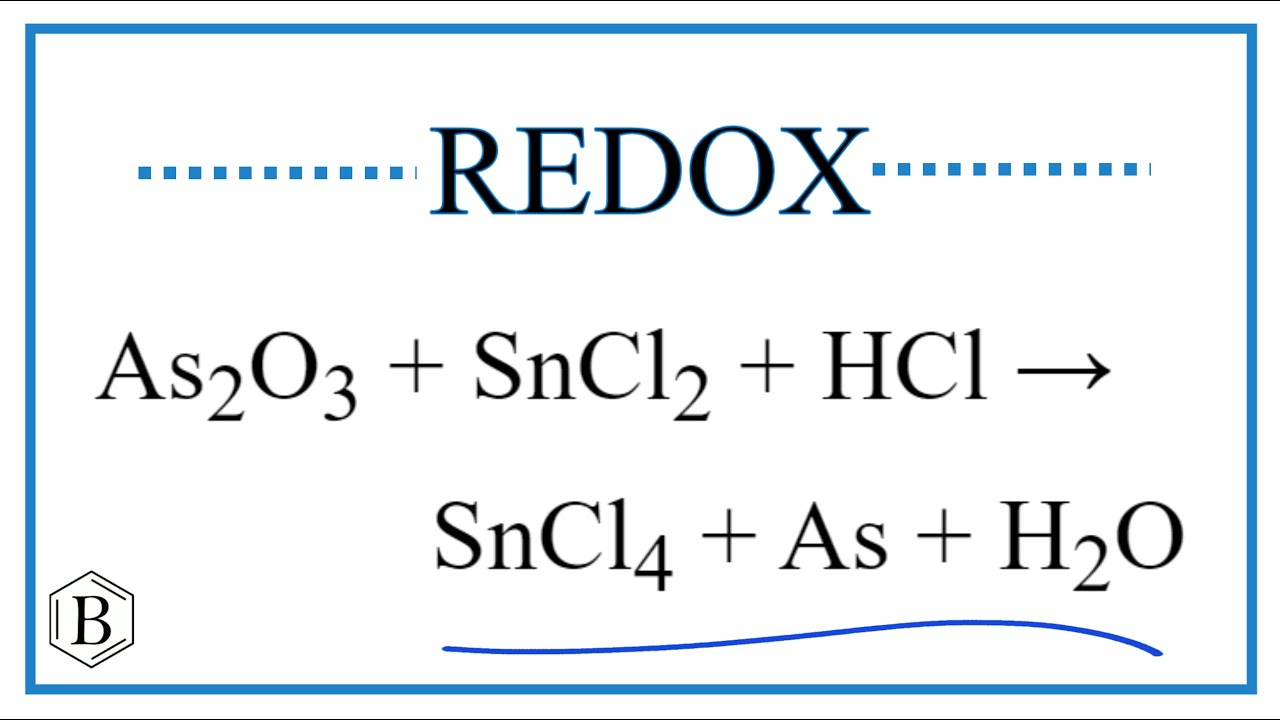
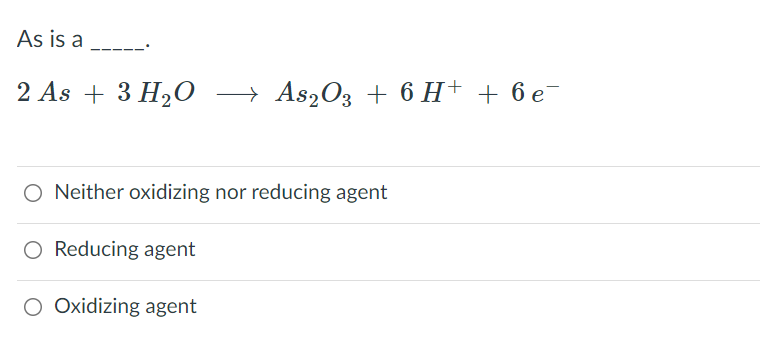SOLVED: Balance the chemical equation by adding coefficients as needed. Equation: HCl(aq) + As2O3(s) + NaNO2(aq) + H2O(l) â†' NO(g) + H3AsO4(aq) + NaCl(aq)

Fundamental Study on Arsenic(III) Halides (AsX3; X = Br, I) toward the Construction of C3-Symmetrical Monodentate Arsenic Ligands | Inorganic Chemistry
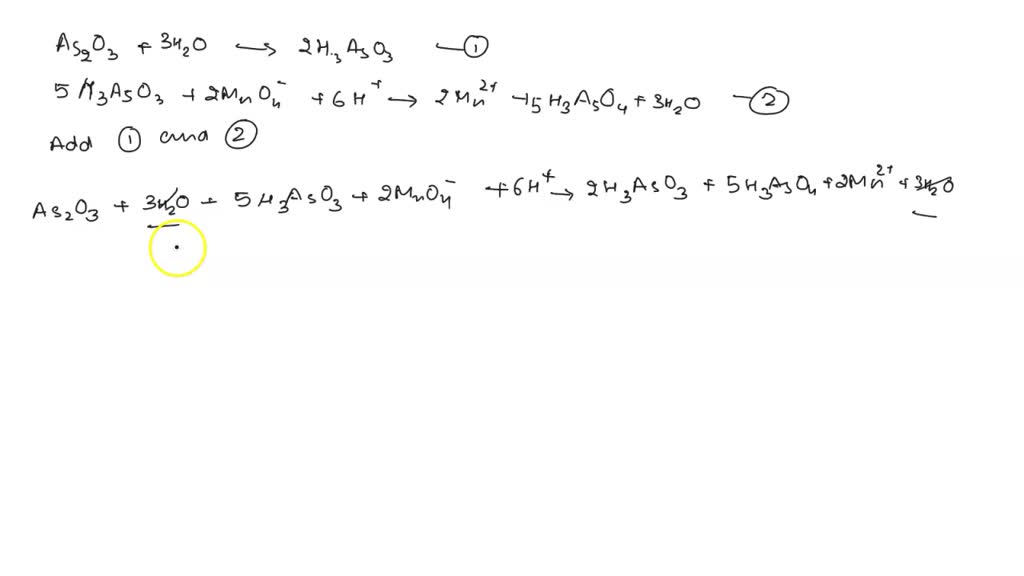
SOLVED: Write the balanced equation between KMnO4 and As2O3. You will have to combine reactions 1 and reaction 4. As2O3 + 3 H2O â†' 2 H3AsO3 (Reaction 1) 5 H3AsO3 + 2
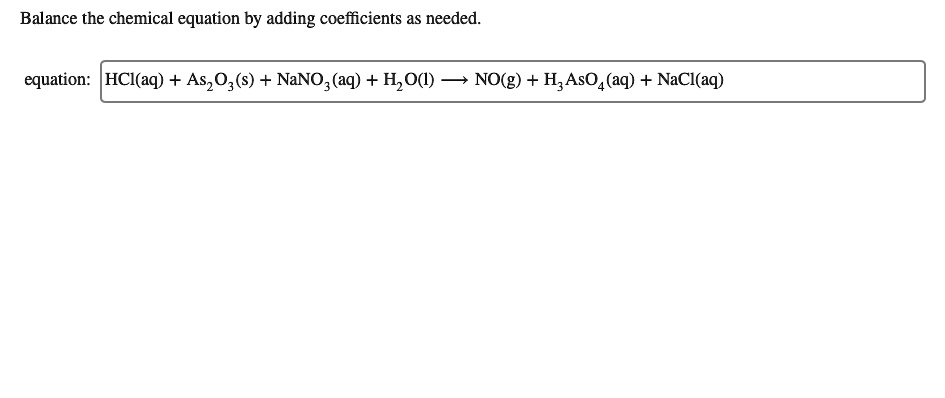
SOLVED: Balance the chemical equation by adding coefficients as needed. Equation: HCl(aq) + As2O3(s) + NaNO2(aq) + H2O(l) â†' NO(g) + H3AsO4(aq) + NaCl(aq)

SOLVED: 7-12. Arsenic(III) oxide (As2O3) is available in pure form and is a useful (but carcinogenic) primary standard for oxidizing agents such as MnO4. The As2O3 is dissolved in base and then

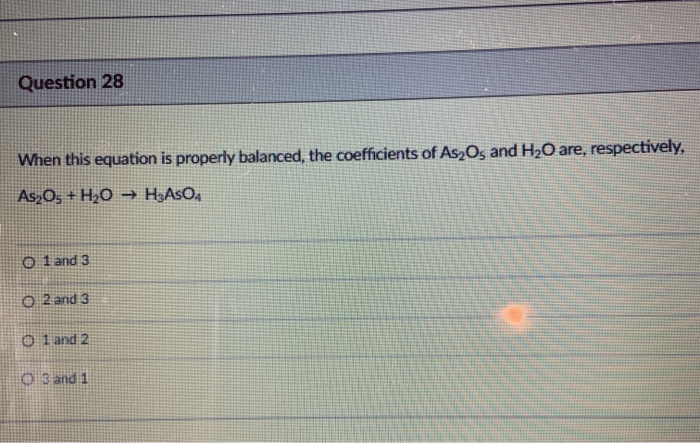
SOLVED: When this equation is properly balanced, the coefficients of As2O3 and H2O are, respectively, As2O3 + H2O â†' HAsO3 + ? 0l4 and ? 0l2 and ? Oland 2 Ol3ald 1
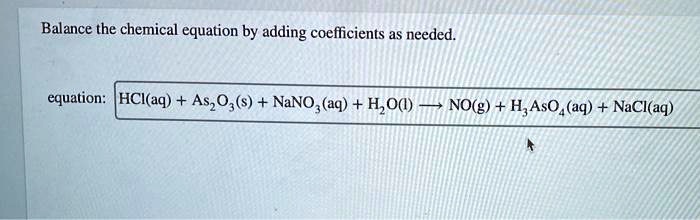
SOLVED: Balance the chemical equation by adding coefficients as needed. equation: HCl(aq) + As2O3(s) + NaNO3(aq) + H2O(l) â†' NO(g) + H3AsO4(aq) + NaCl(aq)
24. In the following reaction(unbalanced) n factor of As2S3 is As2S3 + H+ + NO3 1 NO + H2O + AsO4 3 + S04 2 (2) 4 (3) 24 (4) 28





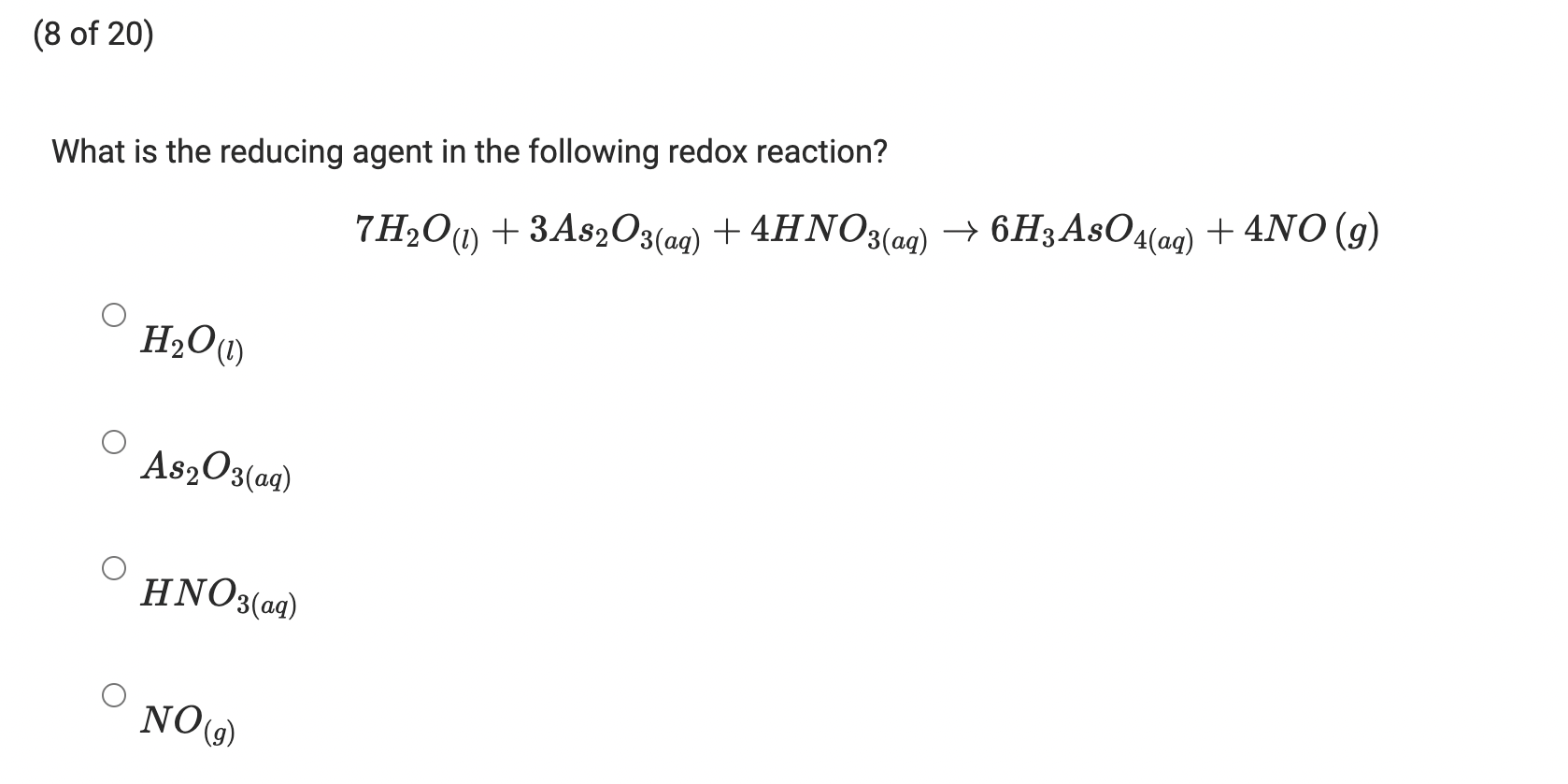
![ANSWERED] A primary standard grade As2O3 M M 197 84... - Physical Chemistry - Kunduz ANSWERED] A primary standard grade As2O3 M M 197 84... - Physical Chemistry - Kunduz](https://media.kunduz.com/media/sug-question-candidate/20210920005325449887-2402955.jpg)