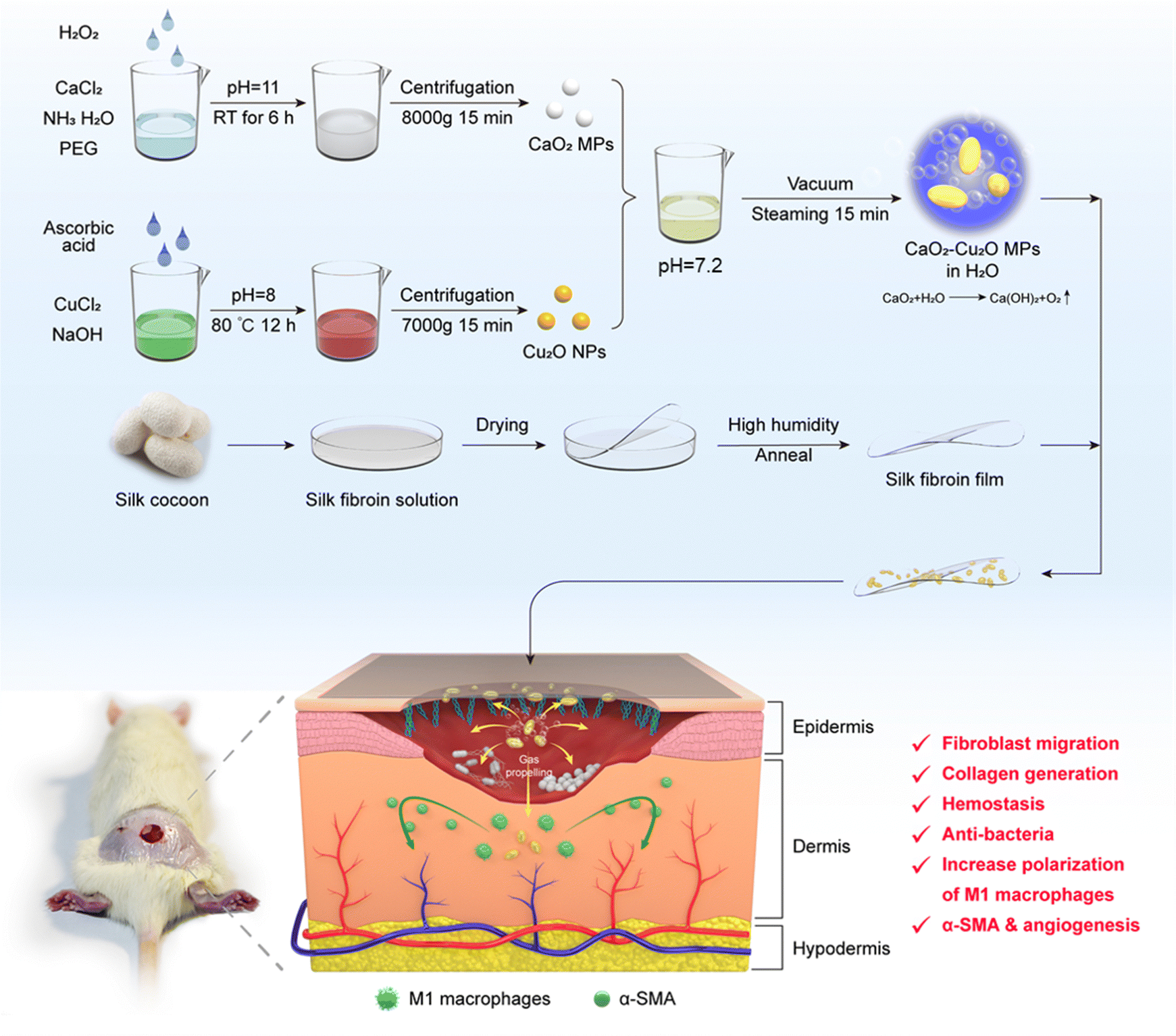
CaO 2 –Cu 2 O micromotors accelerate infected wound healing through antibacterial functions, hemostasis, improved cell migration, and inflammatory reg ... - Journal of Materials Chemistry B (RSC Publishing) DOI:10.1039/D3TB02335D

SOLVED: Consider the combustion of Ca. What are the expected products of the reaction? Select all that apply: CaO2 CaZo H2O CaO CO2

When heated, metal hydroxides decompose to produce a metal oxide and water. Selected the correct balanced - brainly.com

Steady release-activation of hydrogen peroxide and molecular oxygen towards the removal of ciprofloxacin in the FeOCl/CaO2 system - ScienceDirect
Consider the following reaction sequence:12.CaCO3(s)+ 2HCI(aq)CaCl2(aq) + CO2(g) + H20heatCaO(s) + H20(g).CaCO3(s)If the percentage yield of the 1st step is 80

Fabricating collagen films with oxygen-release capabilities: 1,7-octadiene PECVD encapsulation of calcium peroxide - American Chemical Society

Self-Supplying of Hydrogen Peroxide/Oxygen Based on CaO2-Co3O4 Cascade Nanoreactors for Cellular Microenvironment Regulation | ACS Applied Nano Materials

Identify the substance oxidized, substance reduced, oxidizing agent and reducing agent in the following: 1 Cl2 + 2NaBr 2NaCl + - Science - Chemical Reactions and Equations - 13638355 | Meritnation.com

Polymers | Free Full-Text | Synthesis of Controlled-Release Calcium Peroxide Nanoparticles Coated with Dextran for Removal of Doxycycline from Aqueous System







![Punjabi] What type of reactions are represented by following equation Punjabi] What type of reactions are represented by following equation](https://static.doubtnut.com/ss/web-overlay-thumb/10335489.webp)


