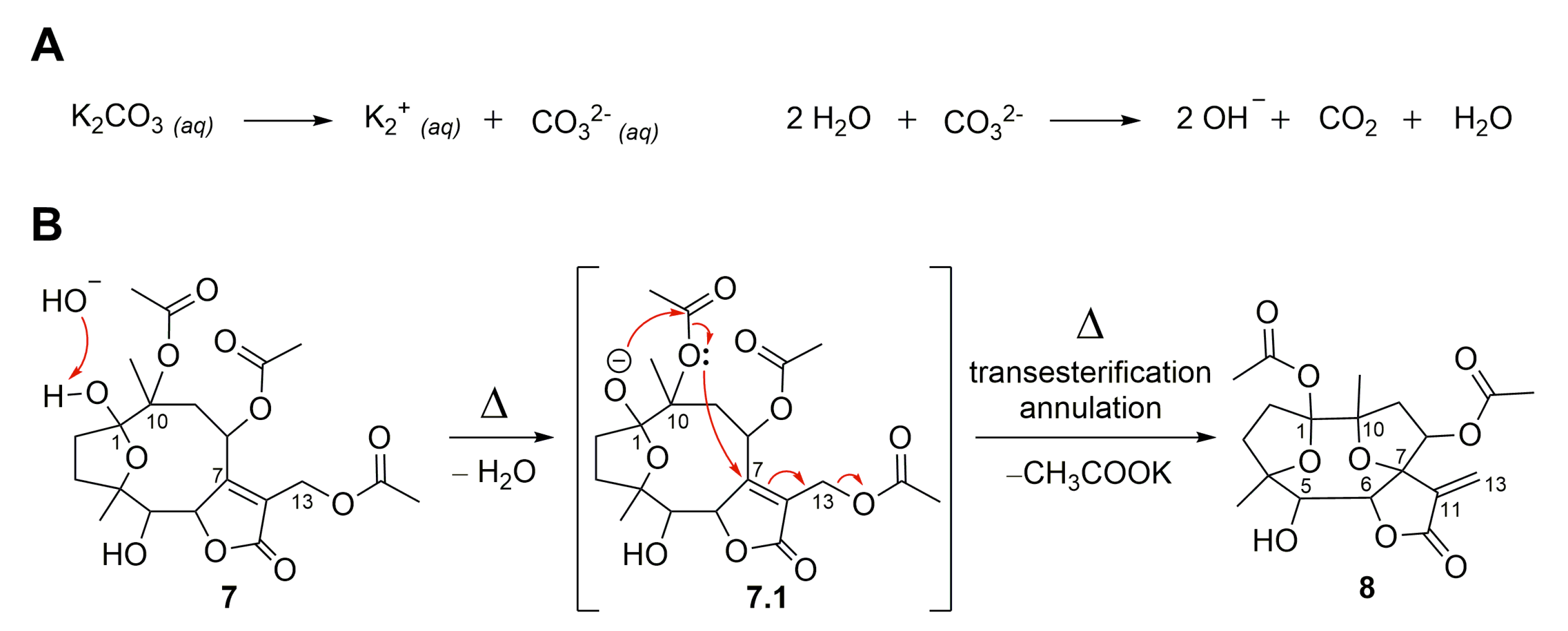
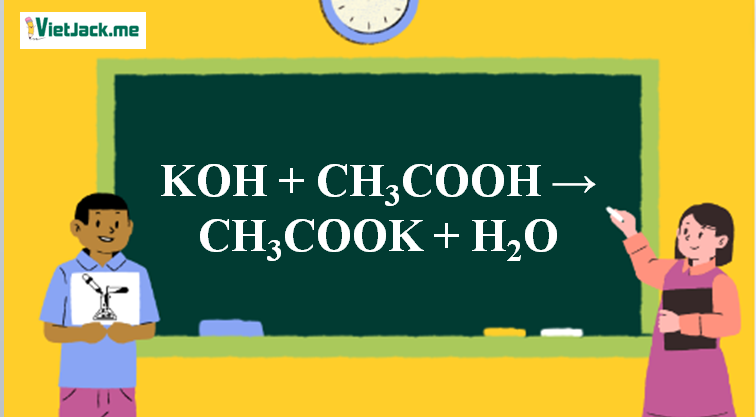Preparation of α-amino LCA derivatives.: Reagents and conditions: a)... | Download Scientific Diagram

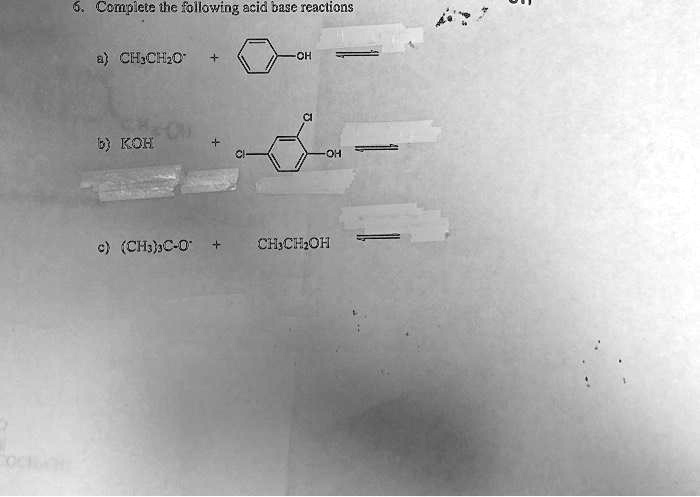
b) Complete the following reactions i) C6H6 + CH3COCI Anhydrous AICI3 > ii) CH3CH2CH2CH,Br + Na dry ether, iii) CH3COOK +H2O electrolysis →
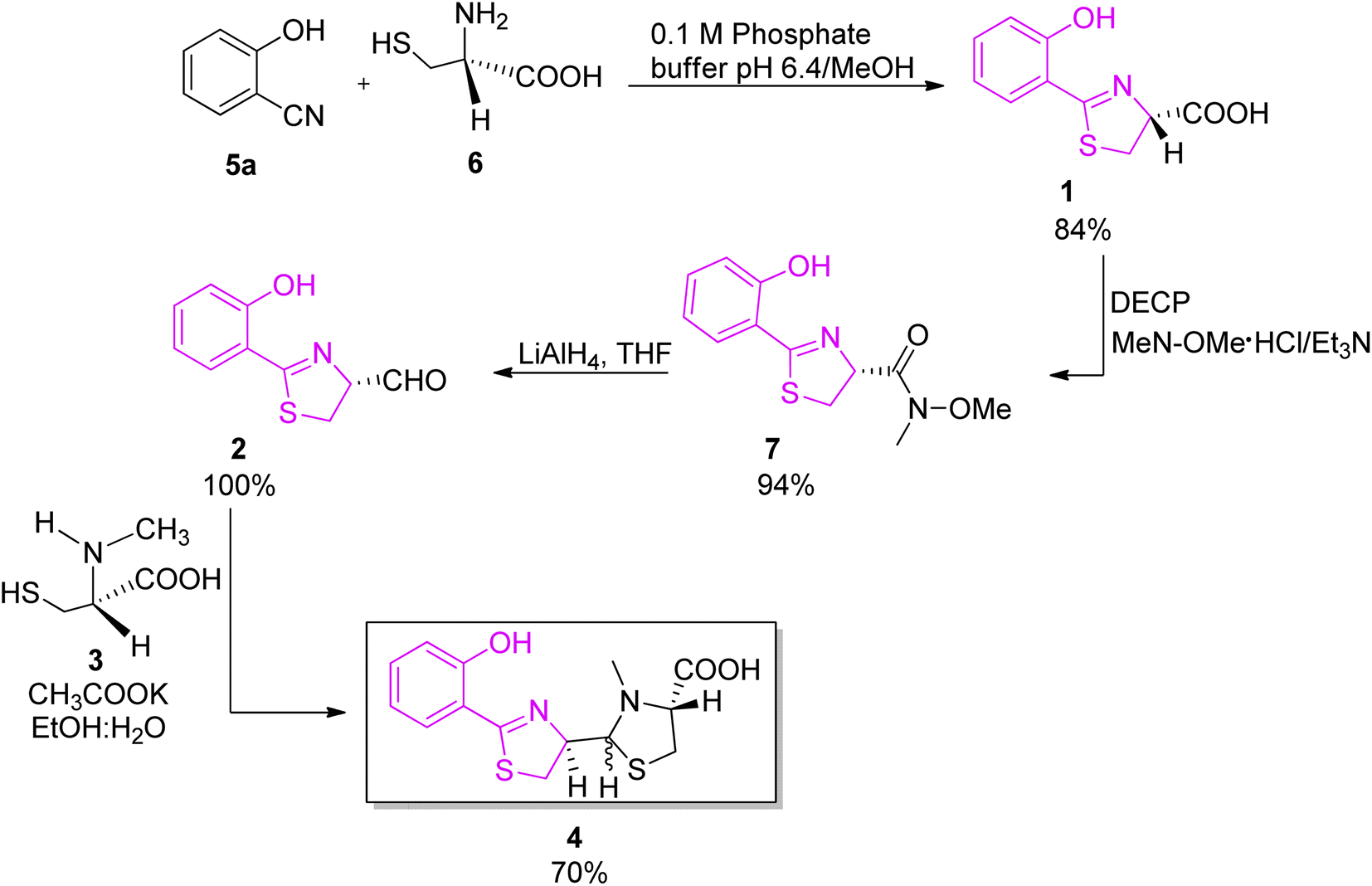
The diversity and utility of arylthiazoline and aryloxazoline siderophores: challenges of total synthesis - RSC Advances (RSC Publishing) DOI:10.1039/D2RA03841B
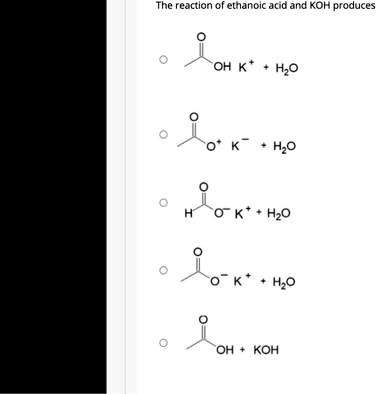
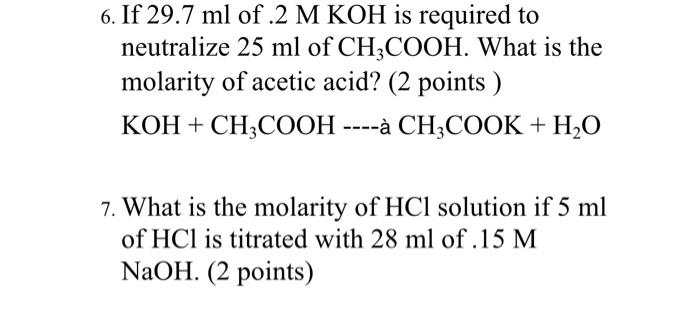
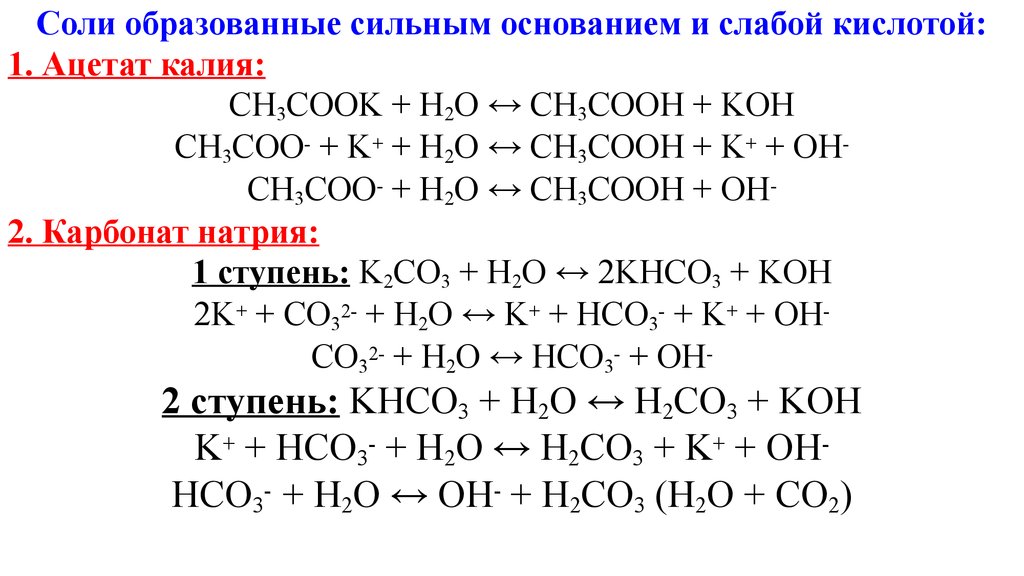
SOLVED: In a reaction between ethanoic acid and KOH, it produces OH- and K+ ions. The balanced chemical equation for this reaction is: CH3COOH + KOH â†' CH3COOK + H2O
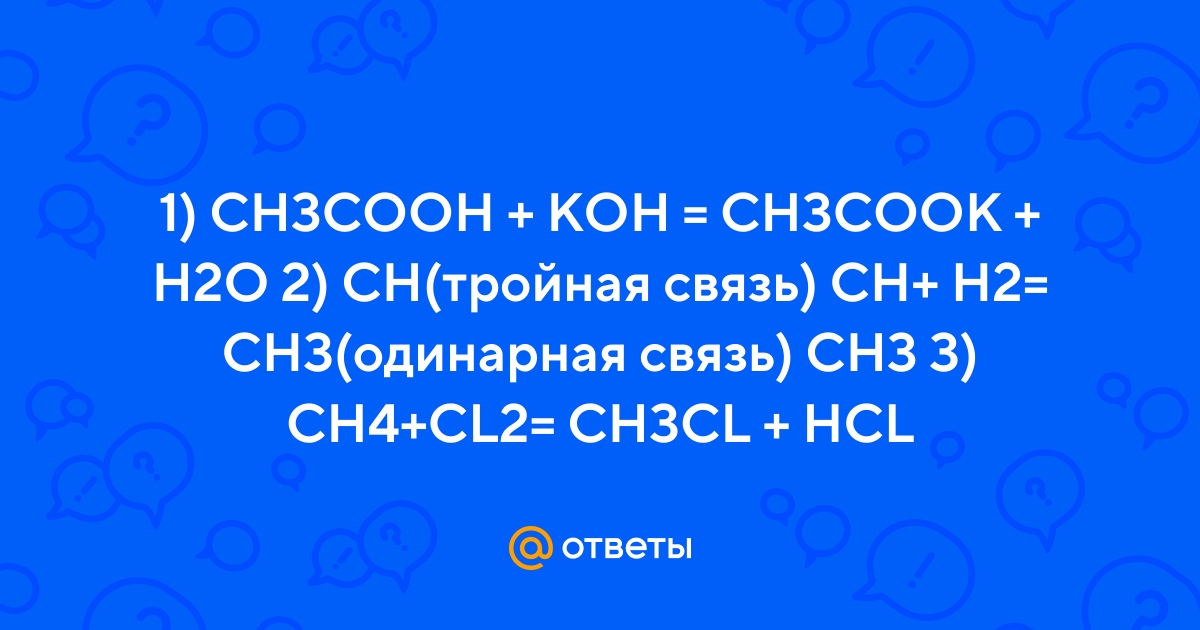
Ответы Mail.ru: 1) CH3COOH + KOH = CH3COOK + H2O 2) CH(тройная связь) CH+ H2= CH3(одинарная связь) CH3 3) CH4+CL2= CH3CL + HCL