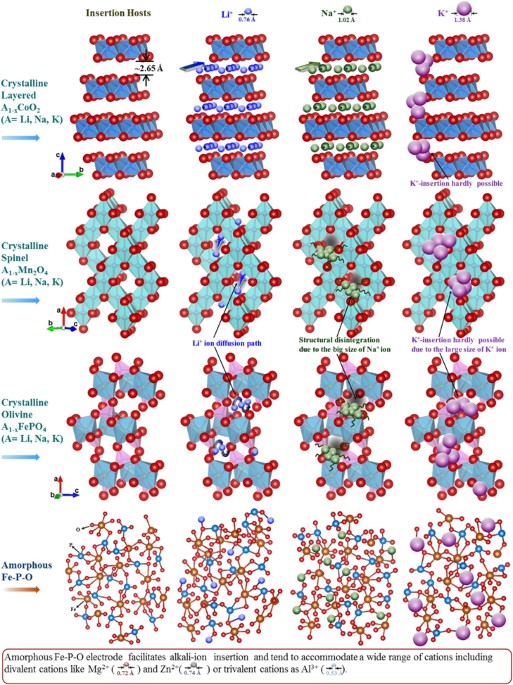
Iron(III) Phosphates Obtained by Thermal Treatment of the Tavorite-Type FePO4·H2O Material: Structures and Electrochemical Properties in Lithium Batteries | Inorganic Chemistry
Synthesis of FePO4 Precursor for LiFePO4 Battery Cathode from Used Nickel Plated A3 Steel Battery Shell by Hydrometallurgy Proce

Iron(III) Phosphates Obtained by Thermal Treatment of the Tavorite-Type FePO4·H2O Material: Structures and Electrochemical Properties in Lithium Batteries | Inorganic Chemistry

Structural and Electrochemical Study of a New Crystalline Hydrated Iron(III) Phosphate FePO4·H2O Obtained from LiFePO4(OH) by Ion Exchange | Chemistry of Materials
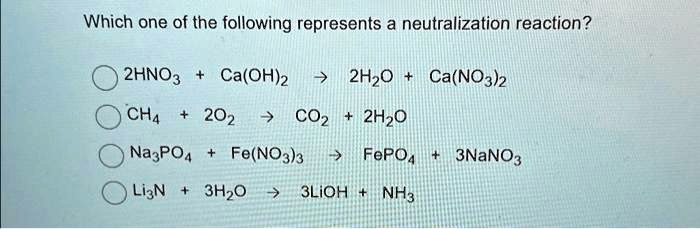
SOLVED: Text: Which one of the following represents a neutralization reaction? 2HNO3 + Ca(OH)2 → H2O + Ca(NO2)2 CH4 + 2O2 → CO2 + 2H2O Na3PO4 + Fe(NO3)3 → FePO4 + 3NaNO3 Li3N + 3H2O → 3LiOH + NH3

Amorphous FePO4/Carbon Nanotube Cathode Preparation via in Situ Nanoprecipitation and Coagulation in a Microreactor | Semantic Scholar

Amorphous FePO4/Carbon Nanotube Cathode Preparation via in Situ Nanoprecipitation and Coagulation in a Microreactor | Semantic Scholar

Iron(III) Phosphates Obtained by Thermal Treatment of the Tavorite-Type FePO4·H2O Material: Structures and Electrochemical Properties in Lithium Batteries | Inorganic Chemistry

Schematic of amorphous FePO4 synthesis and electron microscopy studies.... | Download Scientific Diagram
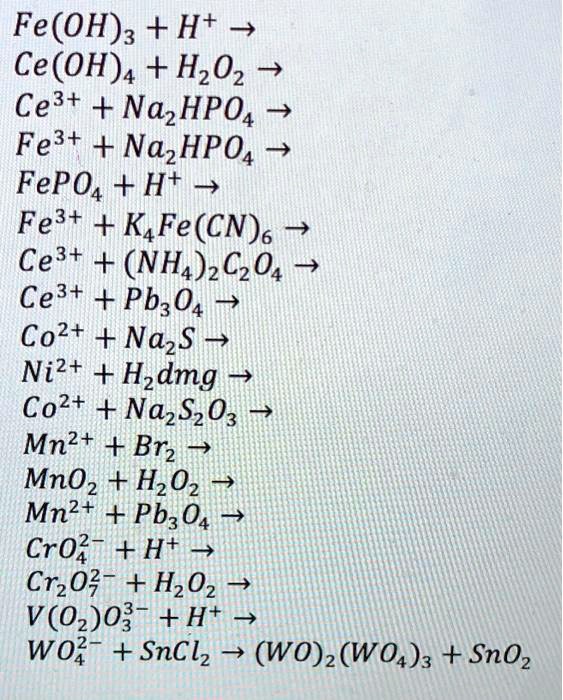
SOLVED: Fe(OH)3 + H2O -> Ce(OH)4 + H2O2 Ce3+ + Na2HPO4 -> Fe3+ + Na2HPO4 4 FePO4 + H2O -> Fe3+ + KFe(CN)6 Ce3+ + (NH4)2C2O4 -> Ce3+ + Pb3O4 Co2+ +
Effects of temperature and phosphoric acid addition on the solubility of iron phosphate dihydrate in aqueous solutions☆ Chines
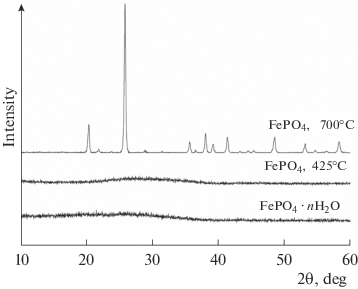
Electrochemical Intercalation of Sodium into Composites Based on Iron(III) Phosphate and Carbon | Inorganic Materials