I) H2O2 + O3 → H2O + 2O2 (II) H2O2 + Ag2O → 2Ag + H2O + O2 Role of hydrogen peroxide in the - Sarthaks eConnect | Largest Online Education Community
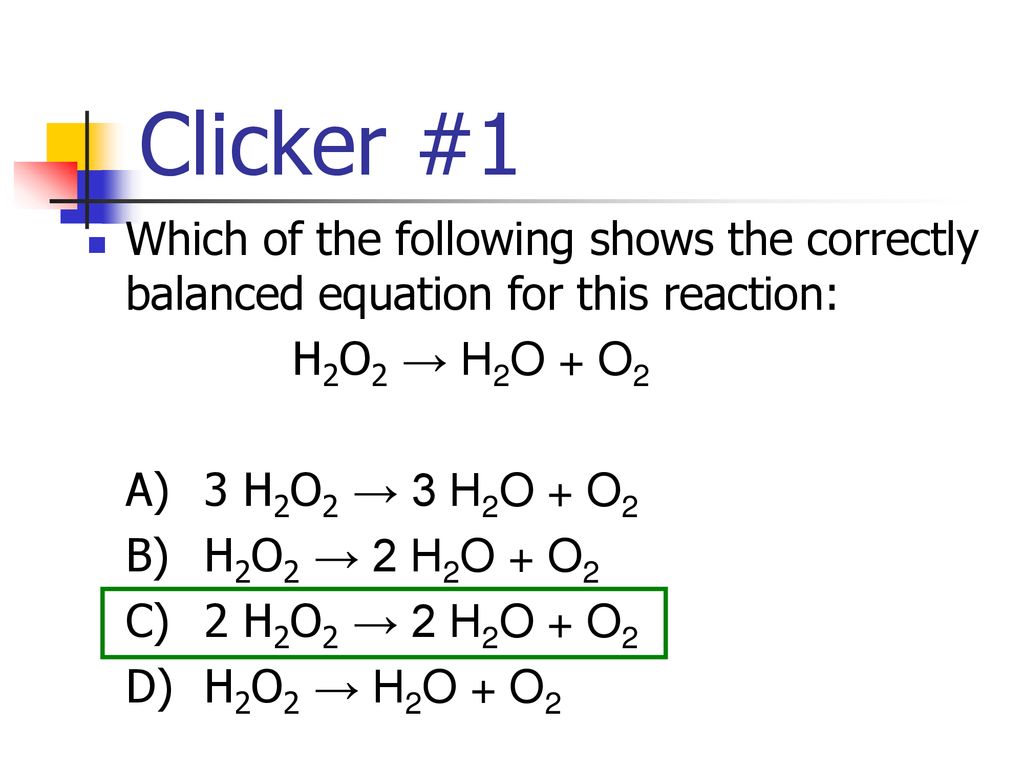
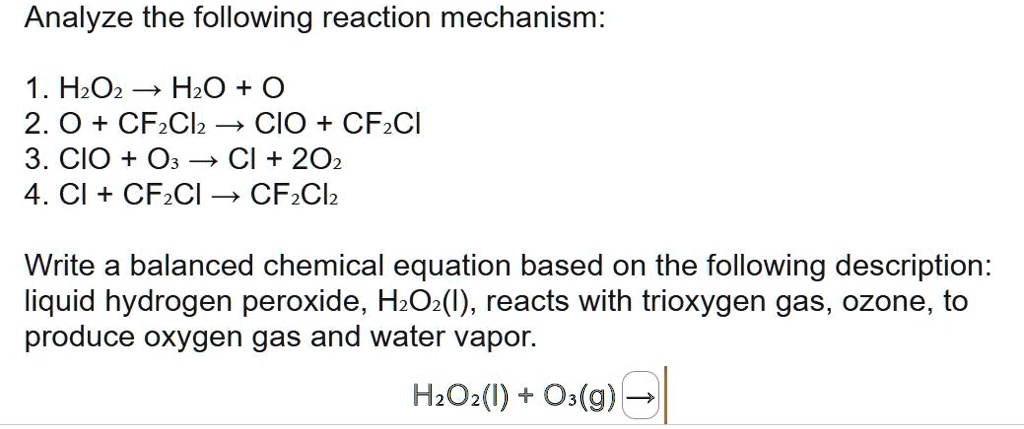
Clicker #1 Which of the following shows the correctly balanced equation for this reaction: H2O2 → H2O + O2 A) 3 H2O2 → 3 H2O + O2 B) H2O2 → 2 H2O + O2. - ppt download

Comment équilibrer : H2O2 → O2 + H2O (peroxyde d'hydrogène, dioxygène, eau) | Physique-Chimie - YouTube

SOLVED: Equations: MnO4 + H2O2 + H2O -> MnO2 + O2 + H2O 2 MnO4 + 6 H2O2 + 5 H2O -> 2 MnO2 + 5 O2 + 8 H2O MnO4 + 8 H+ + Se -> MnO2 + 4 H2O O2 + 3 O2 + 2 e Mn7+ + 2 e -> Mn2+ Mn7+ + Se -> Mn2+

H2O2=H2O+O2 balance the chemical equation @mydocumentary838. h2o2=h2o+o2 balance the equation. - YouTube
Why is the answer B? Can someone explain this to me and why other options are incorrect. I assumed that H2O2 will decompose rapidly to form H20 and O2 with MnO2 as
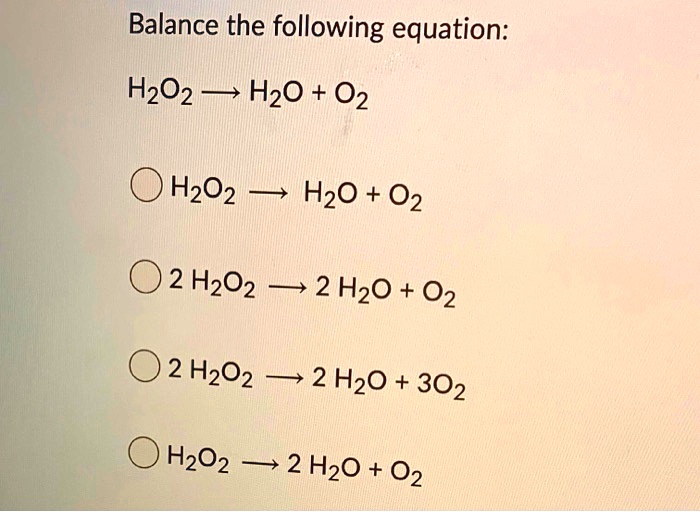
SOLVED: Balance the following equation: H2O2 H2O + O2 H2O2 H2O + O2 2 H2O2 2 H2O + O2 2 H2O2 2 H2O + 3 O2 H2O2 2 H2O + O2

Balance the following chemical equation H2O2+O3⇒H2O+O2 Indicating the changes in oxidation numbers of oxygen, the equivalent weight of H2O2 this reaction.

Kinetic studies on the reaction of 2 with H2O2 in buffered MeCN/H2O... | Download Scientific Diagram
1) H2O2 + O3 → H2O +2O2 2)H2O2 +Ag2O →2Ag +H2O +O2 Determine whether H2O2 is oxidised or reduced in the above reaction? Explain.

Direct production of H2O2 from H2 and O2 in a biphasic H2O/scCO2 system over a Pd/C catalyst: Optimization of reaction conditions - ScienceDirect

Reduction of O2 to H2O and its free radical intermediates (A) Lewis... | Download Scientific Diagram