Eutectic Temperature, Density, and Solubility of H3BO3–H2O, Na2B4O7–H2O, and NaBO2–H2O Binary Systems | Journal of Chemical & Engineering Data
Borazine (B3N3H6) hydrolyses slowly according to following reaction: B3N3H6 + H2O(excess)= NH3 + H3BO3 + H2. Weight of borazine required to form 0.2 mol H3BO3 is
![Write the reaction of action of heat on boric acid [H3BO3]? - Find 2 Answers & Solutions | LearnPick Resources Write the reaction of action of heat on boric acid [H3BO3]? - Find 2 Answers & Solutions | LearnPick Resources](https://www.learnpick.in/files/answerimages/5eb22de957ba09459f6fe1c23b3b4438.jpg)
Write the reaction of action of heat on boric acid [H3BO3]? - Find 2 Answers & Solutions | LearnPick Resources

Eutectic Temperature, Density, and Solubility of H3BO3–H2O, Na2B4O7–H2O, and NaBO2–H2O Binary Systems | Journal of Chemical & Engineering Data

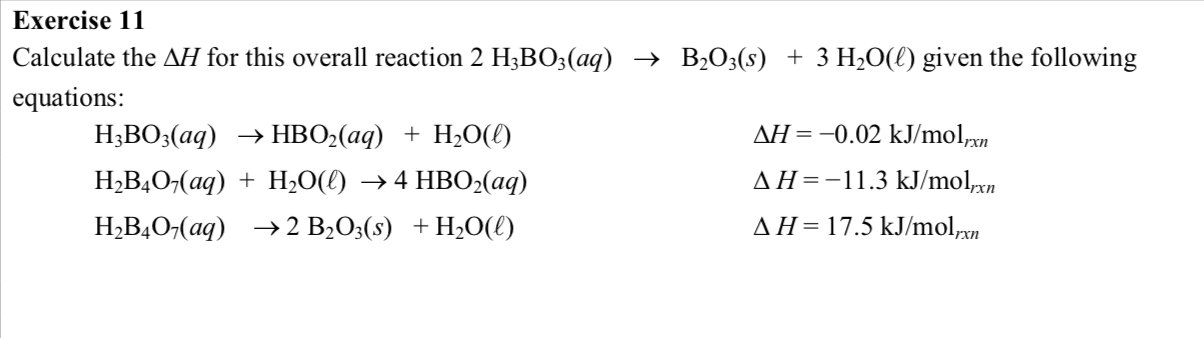
Calculate AH° the following reactions - 2H3BO3(aq) → B2O3(s) + 3H2O() Given that - (a) H3BO3(aq) → HBO2(aq) + H2O(), AHỤ = - 0.02 kJ (b) H2B4076) ▻ 2B0318) + H2O(, AH' =

B2O3 + 3 H2O ---> 2 H3BO3 If diboron trioxide is reacted with water, the product is boric acid. What mass of boric acid is obtained from the full reaction. - ppt video online download

B2O3 + 3 H2O ---> 2 H3BO3 If diboron trioxide is reacted with water, the product is boric acid. What mass of boric acid is obtained from the full reaction. - ppt video online download