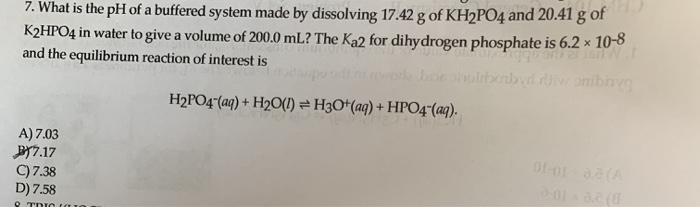KH2PO4 crystallisation from potassium chloride and ammonium dihydrogen phosphate – topic of research paper in Chemical sciences. Download scholarly article PDF and read for free on CyberLeninka open science hub.

Calculate the pH of a buffer solution obtained by dissolving 25.0 g of KH2PO4(s) and 38.0 g of Na2HPO4(s) in water and then diluting to 1.00 L. | Homework.Study.com

Jual POTASSIUM DIHIDROGEN PHOSPHATE ANHIDRAT ( KH2PO4.H2O ) PRO ANALISA 10 GRAM MERCK BEST SELLER | Shopee Indonesia
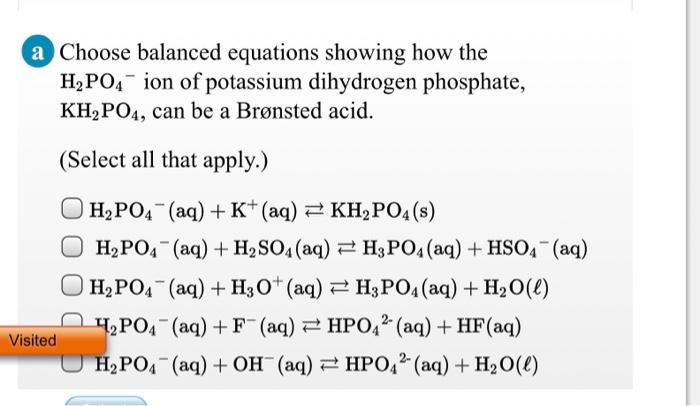
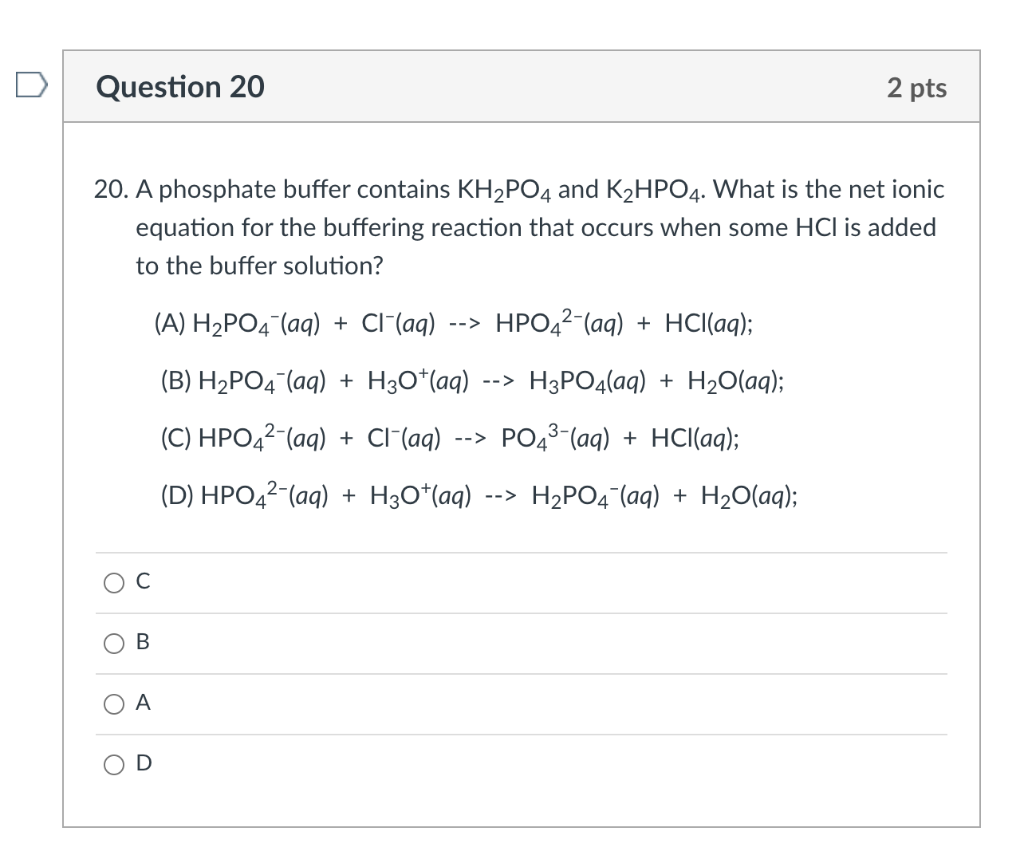
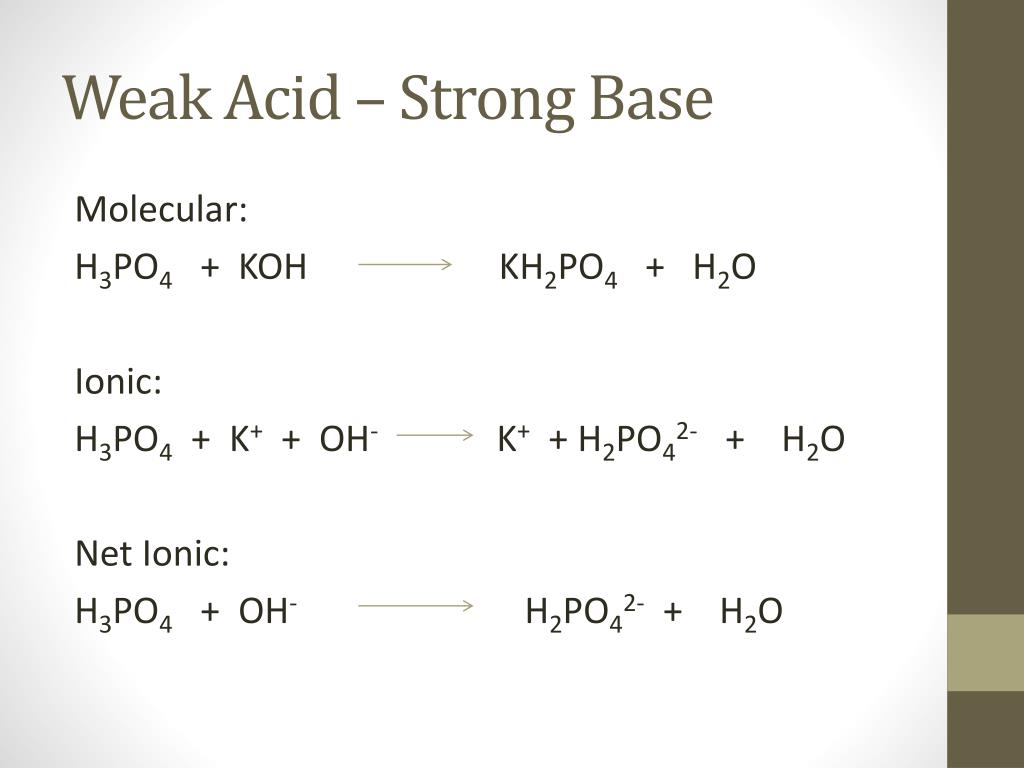
SOLVED: Write the equation and the reaction of the buffer solution KH2PO4 /K2HPO4 when NaOH and HCl is added
Comment calculer le pH d'une solution de KHCO3 (2M) et acide citrique (1M) (1:1) en présence d'un tampon phosphate (PBS : 10mM Na2HPO4 et KH2PO4 1,8 mM) ? - Quora

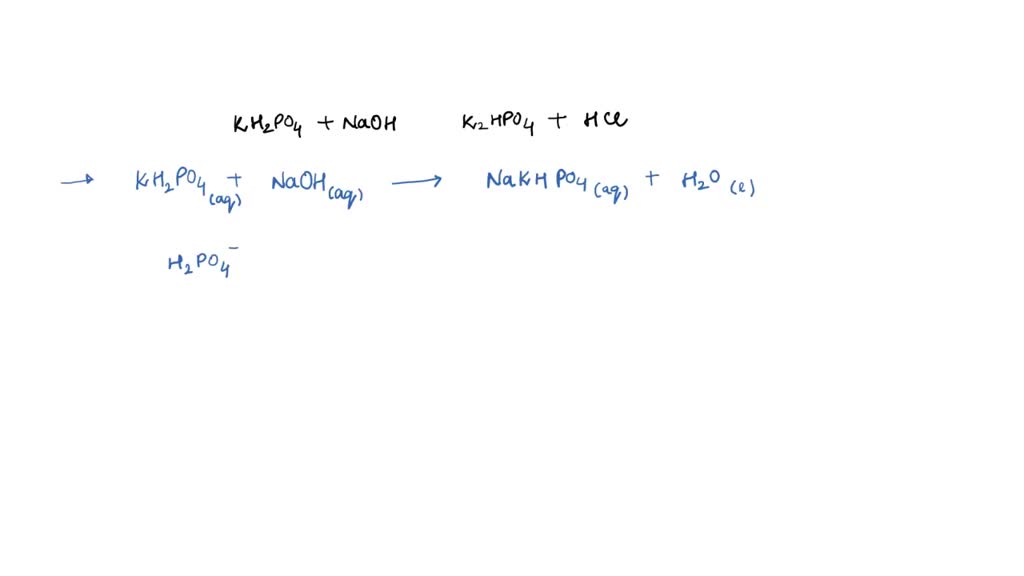
OneClass: Write the chemical reaction for:KH2PO4/K2HPO4 buffer solution + NaOH (aq)andWrite the chemi...

Equilibrium phase diagram of the ternary system KH2PO4 + KNO3 + H2O at... | Download Scientific Diagram

Equilibrium phase diagram of the ternary system KH2PO4 + KNO3 + H2O at... | Download Scientific Diagram

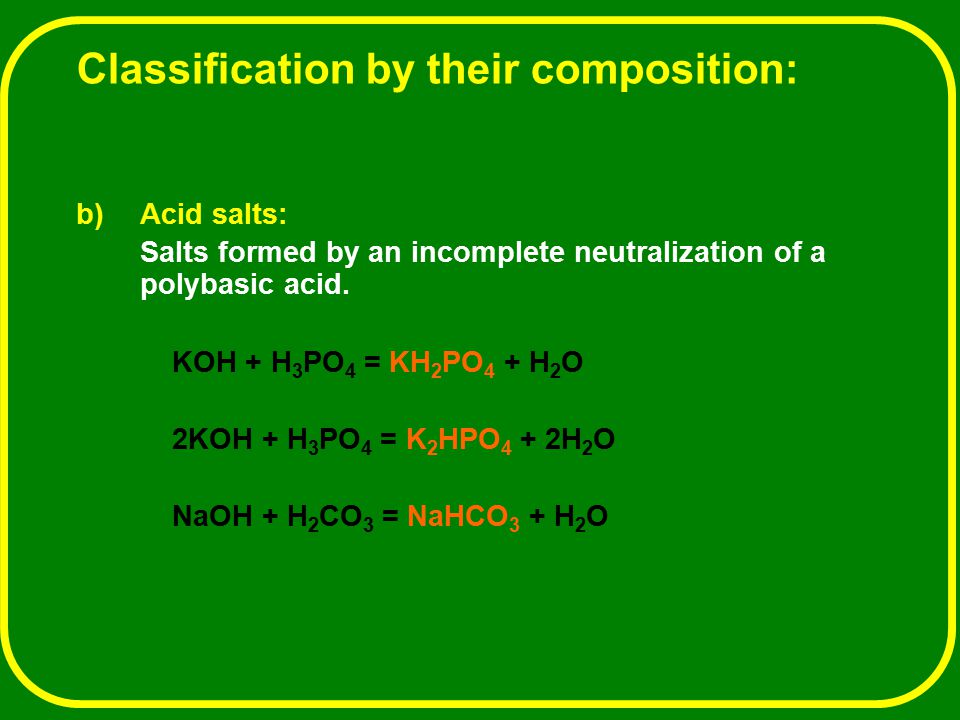
A 0.492 g of KH_2PO_4 is titrated against a solution of 0.112 M NaOH. The volume of the base required to do this is 25.6 mL. The reaction involved is, KH_2PO_4 +

Thermodynamic Properties Data of Ternary System KBr–KH2PO4–H2O at 298.15 K | Journal of Chemical & Engineering Data

SRL Potassium Dihydrogen Orthophosphate for molecular biology, 99.5% 500Gm, CAS 7778-77-0, Molecular Formula : KH2PO4, Storage : Room Temperature, Shelf Life : 60 Months for laboratory use only : Amazon.in: Industrial & Scientific
![SOLVED: Describe stepwise how you would prepare 0.5 L of a 0.15 M phosphate buffer, pH 12.5, using KPO4·H2O (f.w. 230.28) and KH2PO4 (f.w. 174.18). [A-] pH = pKa + log [HA] SOLVED: Describe stepwise how you would prepare 0.5 L of a 0.15 M phosphate buffer, pH 12.5, using KPO4·H2O (f.w. 230.28) and KH2PO4 (f.w. 174.18). [A-] pH = pKa + log [HA]](https://cdn.numerade.com/ask_images/84c1fd22ef3046d0a647ee2adee981f8.jpg)