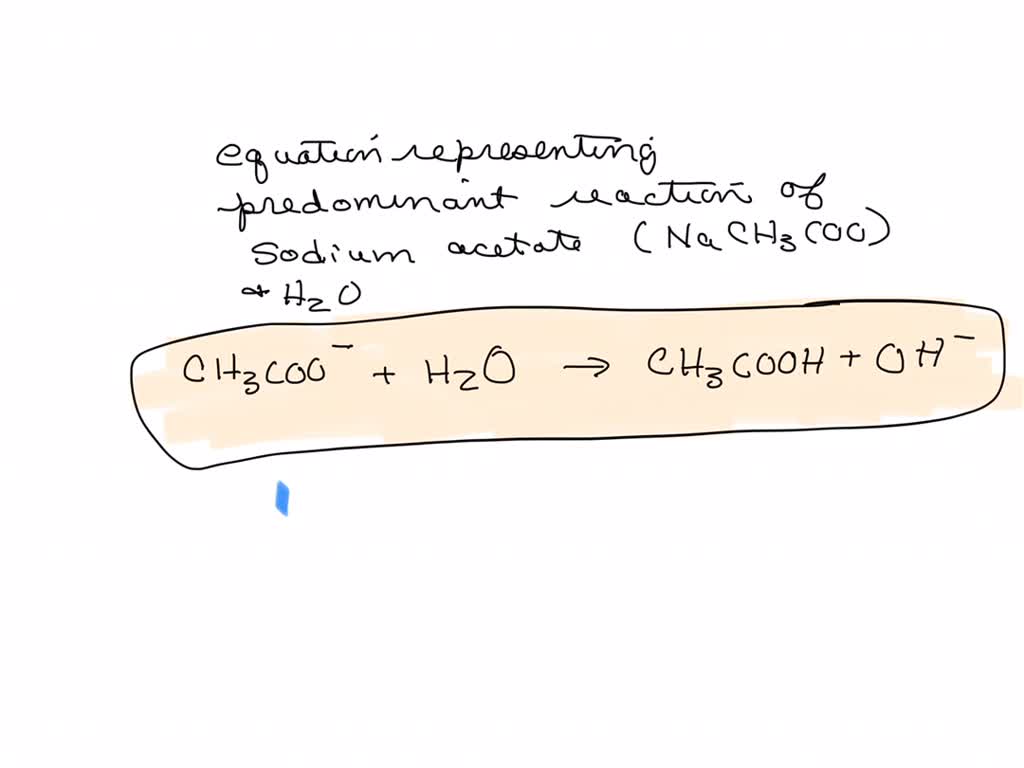
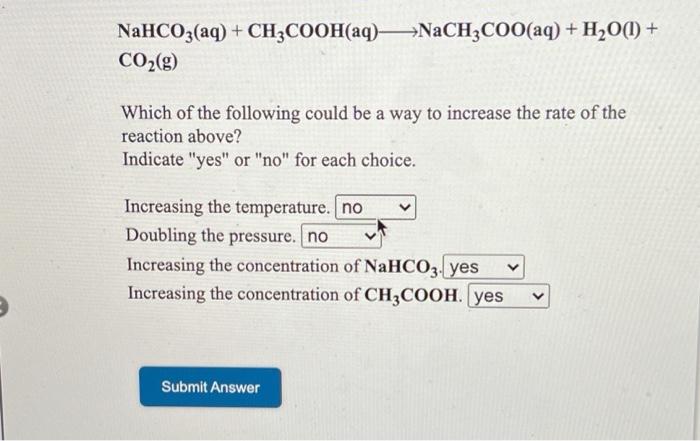
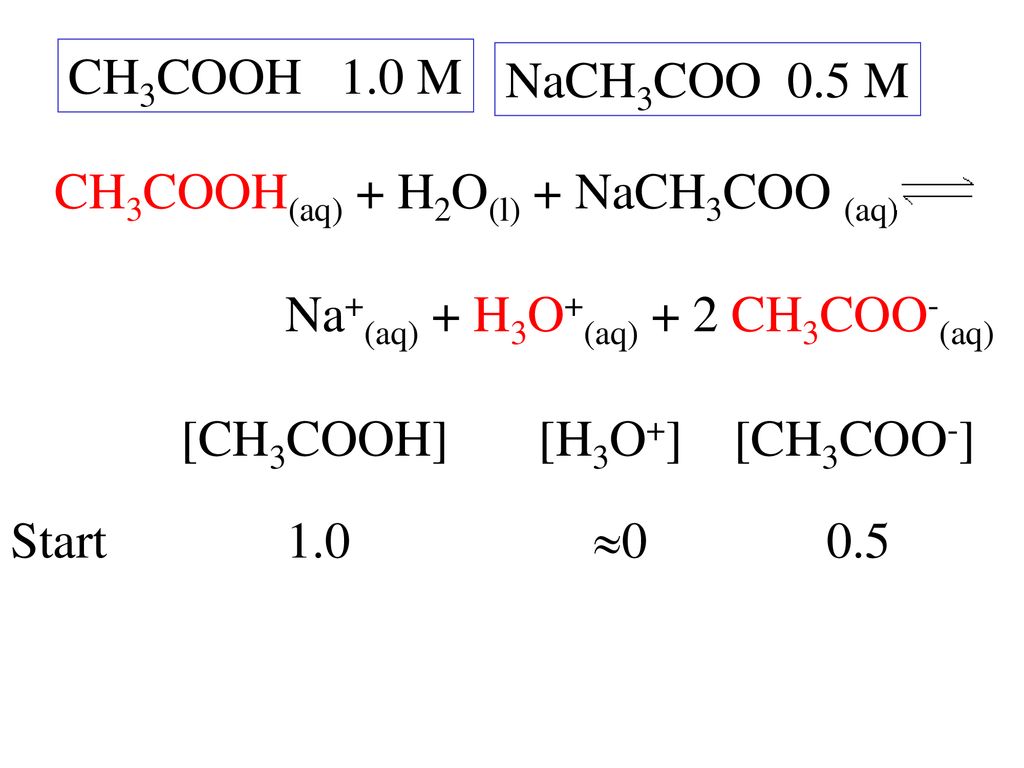
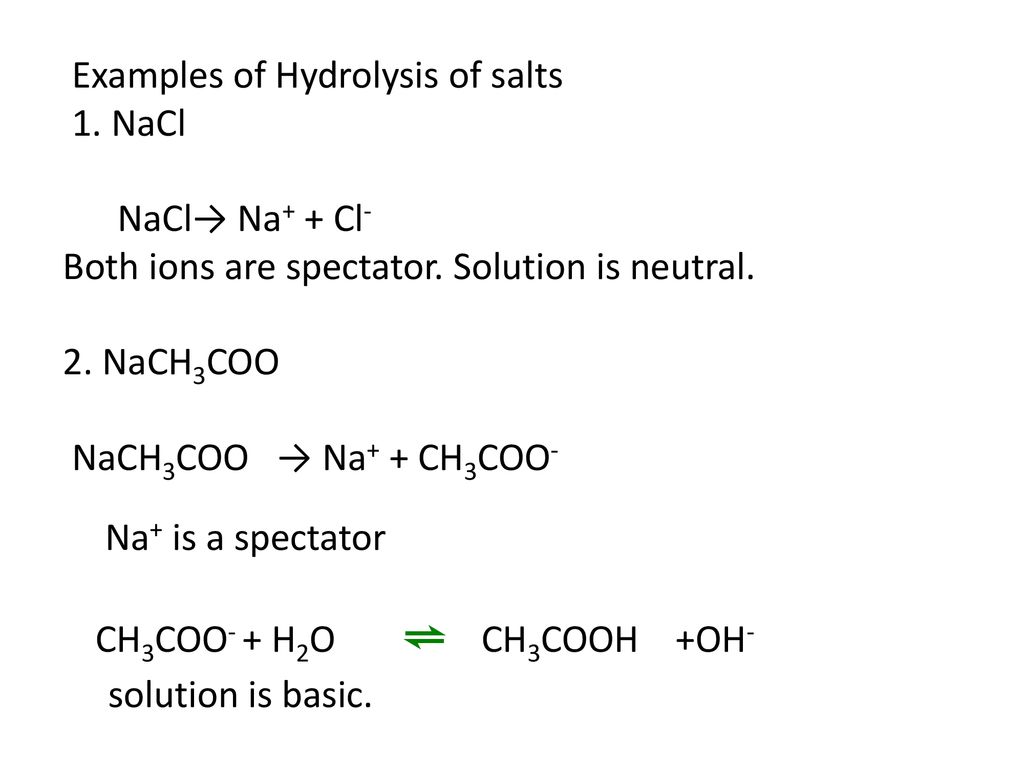
SOLVED: The equation representing the predominant reaction of sodium acetate, NaCH3COO, with water is: CH3COO- + H2O ⇌ CH3COOH + OH- CH3COO- + H2O ⇌ CH3COOH + OH- CH3COOH + H2O ⇌
Why does the solution of sodium acetate give more concentration of Hydroxide ion? Shouldn't the number of Hydroxide ion and hydrogen ion be equal? - Quora
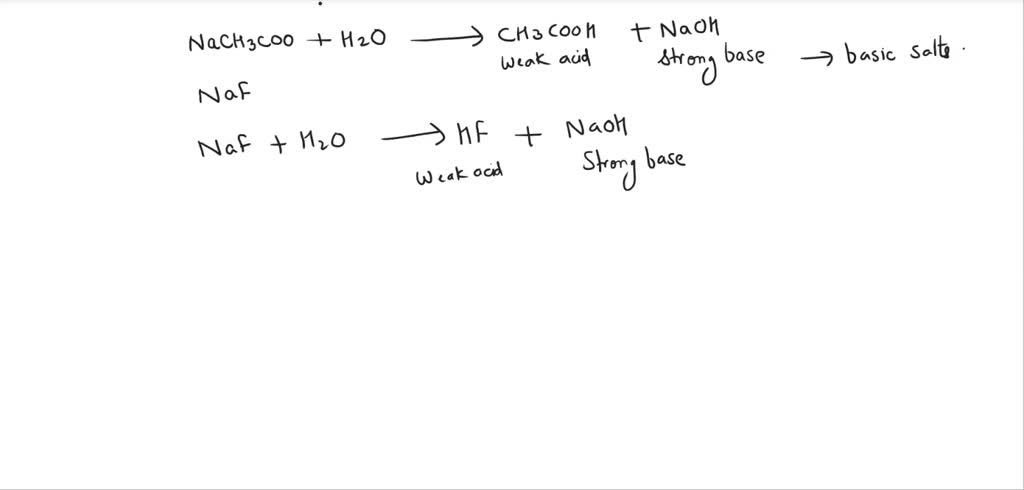
SOLVED: Write chemical equations to show that NaCH3COO and NaF are basic salts. Indicate which of the two salts are more basic in aqueous solutions, and explain why.


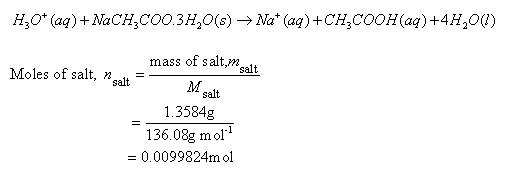





![Sodium Acetate Trihydrate [CH3COONa.3H2O] Molecular Weight Calculation - Laboratory Notes Sodium Acetate Trihydrate [CH3COONa.3H2O] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2022/12/sodium-acetate-trihydrate-molecular-weight-calculation-300x228.jpg)


